Paclitaxel is the world’s best-selling plant-based anticancer drug and one of the most effective anticancer drugs overthe past 30 years. It is widely used in the treatment of various types of cancer, including breast cancer, lung cancer, and ovarian cancer. In the late 1990s and early 21st century, the annual sales of paclitaxel exceeded $1.5 billion and reached $2.0 billion in 2001, making it the best-selling drug in 2001. In 2019, the market for paclitaxel and its derivatives was approximately $15 billion, and it is expected to reach $20 billion by 2025.
As an anticancer drug, the molecular structure of paclitaxel is extremely complex, with highly oxidized, intricate bridged rings and 11 stereocenters, making it widely recognized as one of the most challenging natural products to chemically synthesize. Since the first total synthesis of paclitaxel was reported by the Holton and Nicolaou research groups in 1994, more than 40 research teams have been engaged in the total synthesis of paclitaxel. However, even in the shortest chemical synthesis route to date, the overall yield of paclitaxel is only 0.118%, which falls short of meeting the demand for industrial production. Currently, the industrial production of paclitaxel employs a semi-synthetic strategy: isolating paclitaxel precursors (such as baccatin III) from plant cell cultures or Taxus leaves, and then converting them into paclitaxel through chemical methods. However, the semi-synthetic strategy heavily relies on natural resources and is limited by the slow growth of Taxus cells or leaves, and thus cannot meet the growing market demand.
With the rapid development of biotechnology, synthetic biology strategies for the microbial biosynthesis of plant natural products have emerged as a powerful approach to efficiently produce complex plant natural products. Therefore, achieving efficient, environmentally friendly, and sustainable production of paclitaxel through synthetic biology has attracted widespread attention. However, realizing the de novo synthesis of paclitaxel in a heterologous system requires identifying the key enzymes missing in the paclitaxel biosynthetic pathway and establishing a complete biosynthetic route for paclitaxel.
To address the long-standing challenge of paclitaxel biosynthesis in Taxus, two research teams led by Prof. Jianbin Yan (Agricultural Genomics Institute at Shenzhen, AGIS) and Prof. Xiaoguang Lei (Peking University, PKU), as well as other research teams from other 5 different institutions including Tsinghua University and UCLA have collaborated together to successfully identify the missing enzymes and achieve the reconstitution of the biosynthetic enzymes leading to baccatin III. The researchers utilized a tobacco heterologous expression system to perform activity screening of the CYP725A gene family found specifically in Taxus via substrate co-injection strategy. They have successfully identified a biosynthetic enzyme named Taxane oxetanase (TOT) that catalyzes the formation of the oxetane ring during the taxol biosynthesis pathway, as shown in Fig. 1. TOT catalyzes the formation of the unique oxetane ring through oxidation of the C4,20 double bond and subsequent rearrangement of the adjacent acetyl group at the C5 position, as depicted in Fig. 2. This novel reaction mechanism of oxetane ring formation breaks the conventional understanding that oxetane ring formation in the taxol biosynthesis pathway is achieved through a rearrangement reaction of the corresponding epoxide.
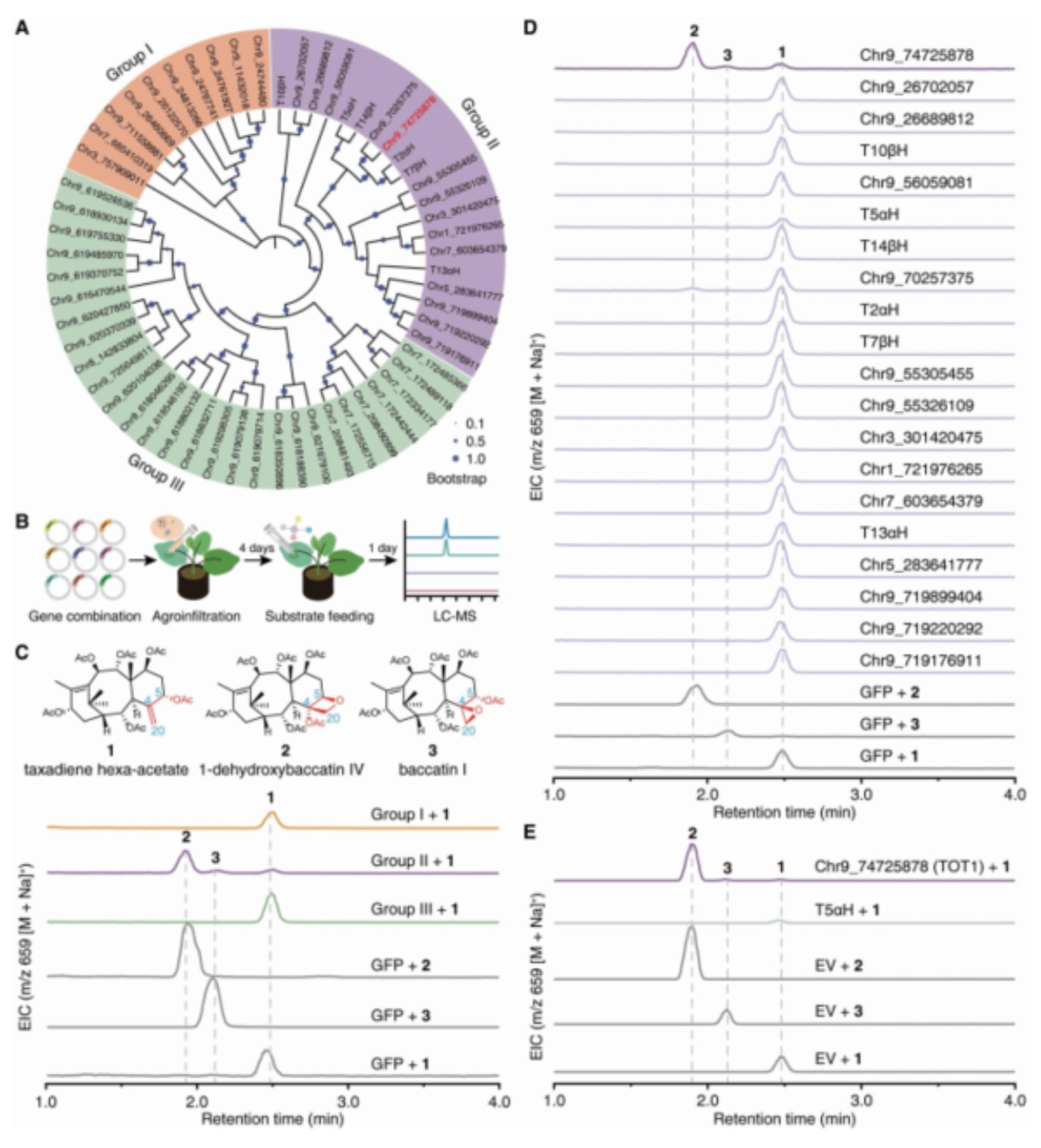
Fig. 1 The identification of Taxane oxetanase (TOT) using substrate/gene co-injection strategy.
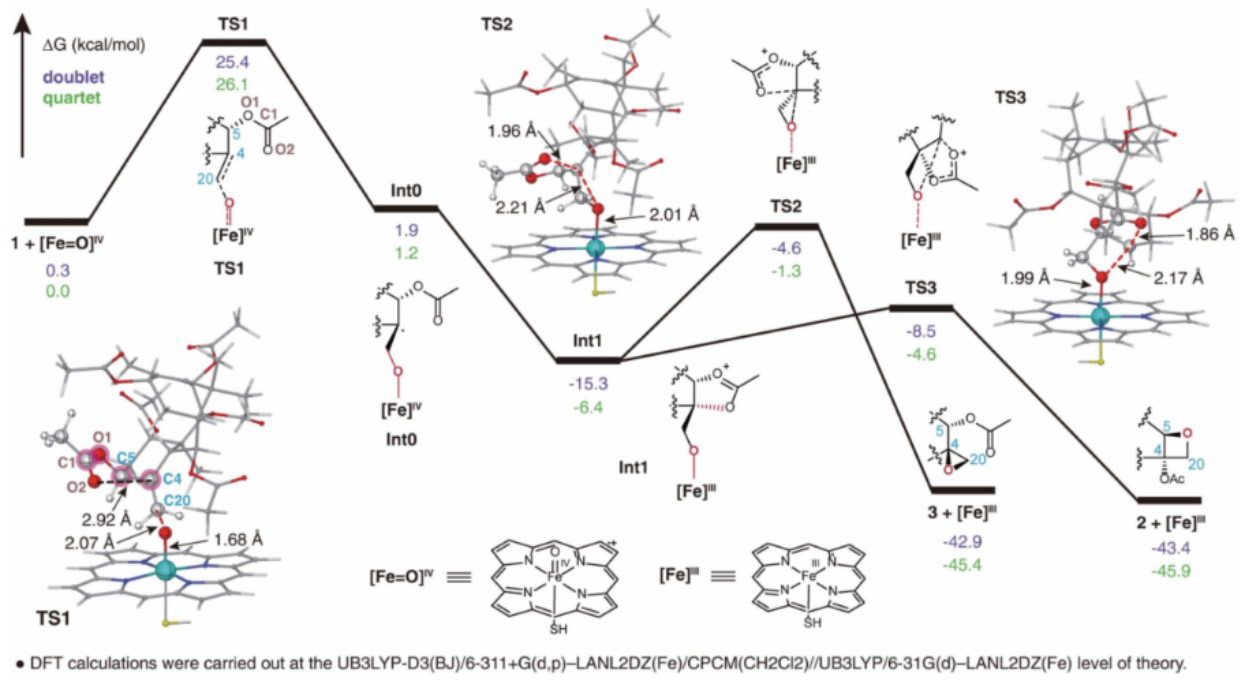
Fig. 2 The reaction mechanism of the TOT-catalyzed oxidation reaction.
Meanwhile, by focusing on the structurally simpler compound, taxusin, the researchers identified 17 candidate genes encoding enzymes responsible for the C9 oxidation of taxanes using the co-expression analysis and metabolism analysis (Fig. 3). These candidate genes were further subjected to activity screening by reconstructingthe taxusin biosynthetic pathway in tobacco, leading to the discovery of the enzyme responsible for C9 oxidation in taxanes (Fig. 3), which was named Taxane 9α hydroxylase (T9αH).
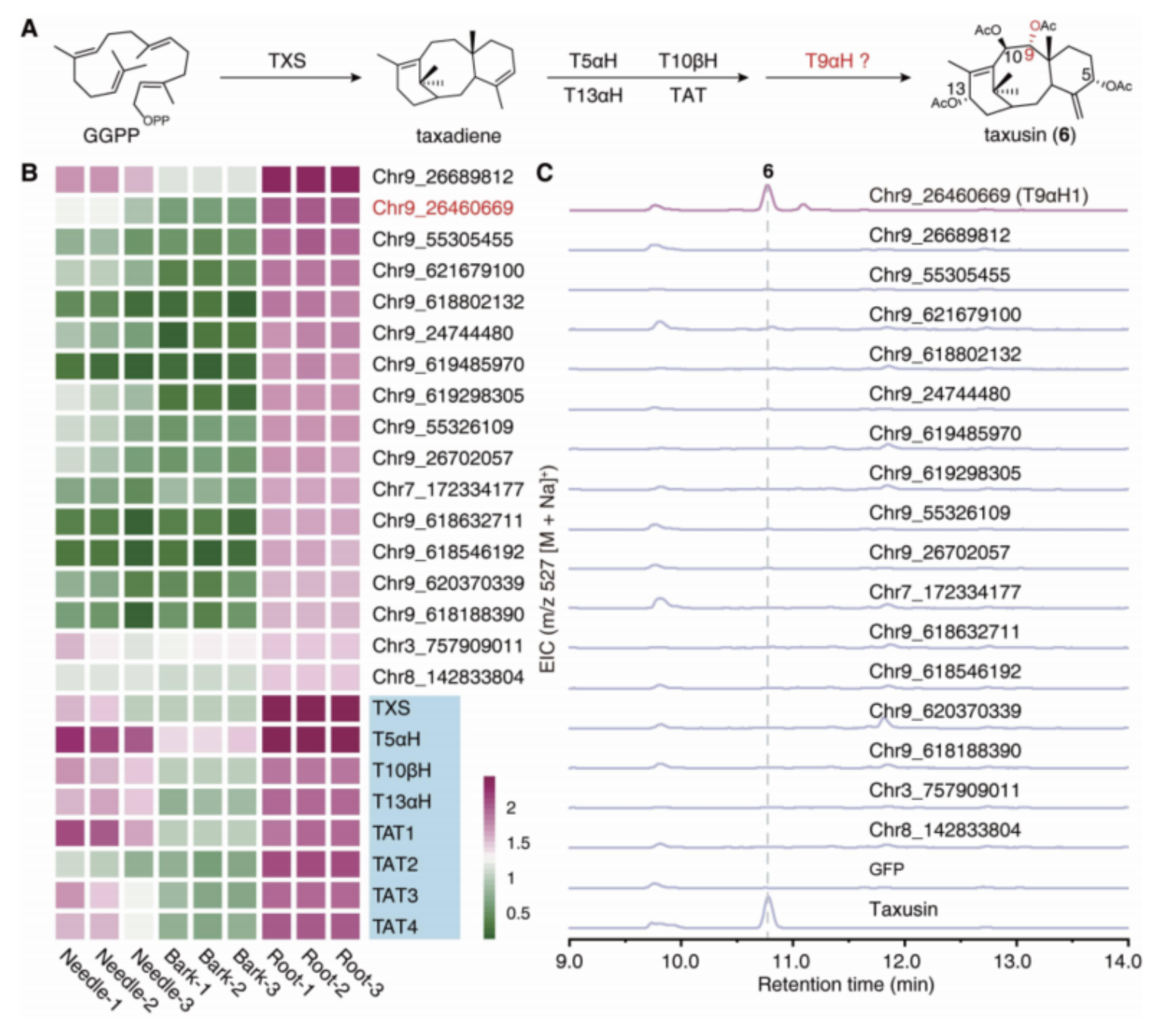
Fig. 3 Activity screening and identification of T9αH.
With these two newly identified enzymes TOT and T9αH in hands, researchers tried to achieve the total biosynthesis of baccatin III in tobacco via co-expressing them with other known biosynthetic genes of taxol. They successfully detected the production of baccatin III in tobacco when TOT and T9αH were co-expressed with other 7 known biosynthetic genes (TXS, T5αH, T13αH, T2αH, T7βH, TAT, and TBT). Furthermore, they have demonstrated that these nine genes are the core genes forbaccatin III biosynthesis since every gene is indispensable for the biosynthesis of baccatin III in tobacco (Fig. 4).
Further biochemical studies showed that these core genes exhibit close functional synergy and are co-regulated by the plant hormone jasmonate, demonstrating similar induction expression patterns and strong expression correlation (Fig. 4). By combining subcellular localization analysis and other experimental results, researchers provide a complete overview of the biosynthetic process of baccatin III.The starting substrate GGPP is catalyzed by TXS to form taxadiene in chloroplasts. Subsequently, taxadiene is transferred to the cytoplasm through the contact sites of plastid–endoplasmic reticulum and undergoes concerted catalysis by six membrane-bound oxidases (T2αH, T5αH, T7βH, T9αH, T13αH, and TOT) anchored in the endoplasmic reticulum and two cytoplasmic acyl transferases (TAT and TBT), ultimately resulting in the formation of baccatin III (Fig. 4).

Fig. 4Reconstitution and bioinformatic analysis of the biosynthetic core genes of baccatin III.
In summary, this study combines multiple omics analysis and extensive functional validation to successfully identify key missing enzymes in the biosynthetic pathway of paclitaxel. It reveals a novel mechanism by which plant cells catalyze the formation of oxetane rings and discovers the shortest route for paclitaxel heterologous biosynthesis. Byco-expressing 9 core enzymes in tobacco, the researchers achieve the bioproduction of paclitaxel precursor baccatin III, laying the foundation for large-scale production of paclitaxel and also providing theoretical guidance for biosynthetic studies on hundreds of other taxane natural products.
Prof. Jianbin Yan from AGIS and Prof. Xiaoguang Lei from the College of Chemistry and Molecular Engineering, PKU are the corresponding authors of the paper. Mr. Bin Jiang (a master's graduate from AGIS), Dr. Lei Gao (a deputy investigator from PKU), Mr. Haijun Wang (a Ph.D. student from PKU), and Miss Yaping Sun (a Ph.D. student from AGIS) are the co-first authors. Prof. Wei Li from AGIS, Prof. Yi Shang from Yunnan Normal University, Prof. K. N. Houk from the University of California, Los Angeles. Prof. Xingyao Xiong from AGIS, Prof. Daoxin Xie from Tsinghua University, and Prof. Sanwen Huang from AGIS have all provided important guidance for this research. This study was financially supported by the Ministry of Science and Technology of the People's Republic of China, the National Natural Science Foundation of China, the National Institutes of Health of the United States, the Chinese Academy of Agricultural Sciences, the Beijing National Laboratory for Molecular Sciences, the Peking University-Tsinghua University Joint Center for Life Sciences Research, the Guangdong Province, Shenzhen Municipality, and Dapeng New District.
Original link for the paper:https://www.science.org/doi/10.1126/science.adj3484