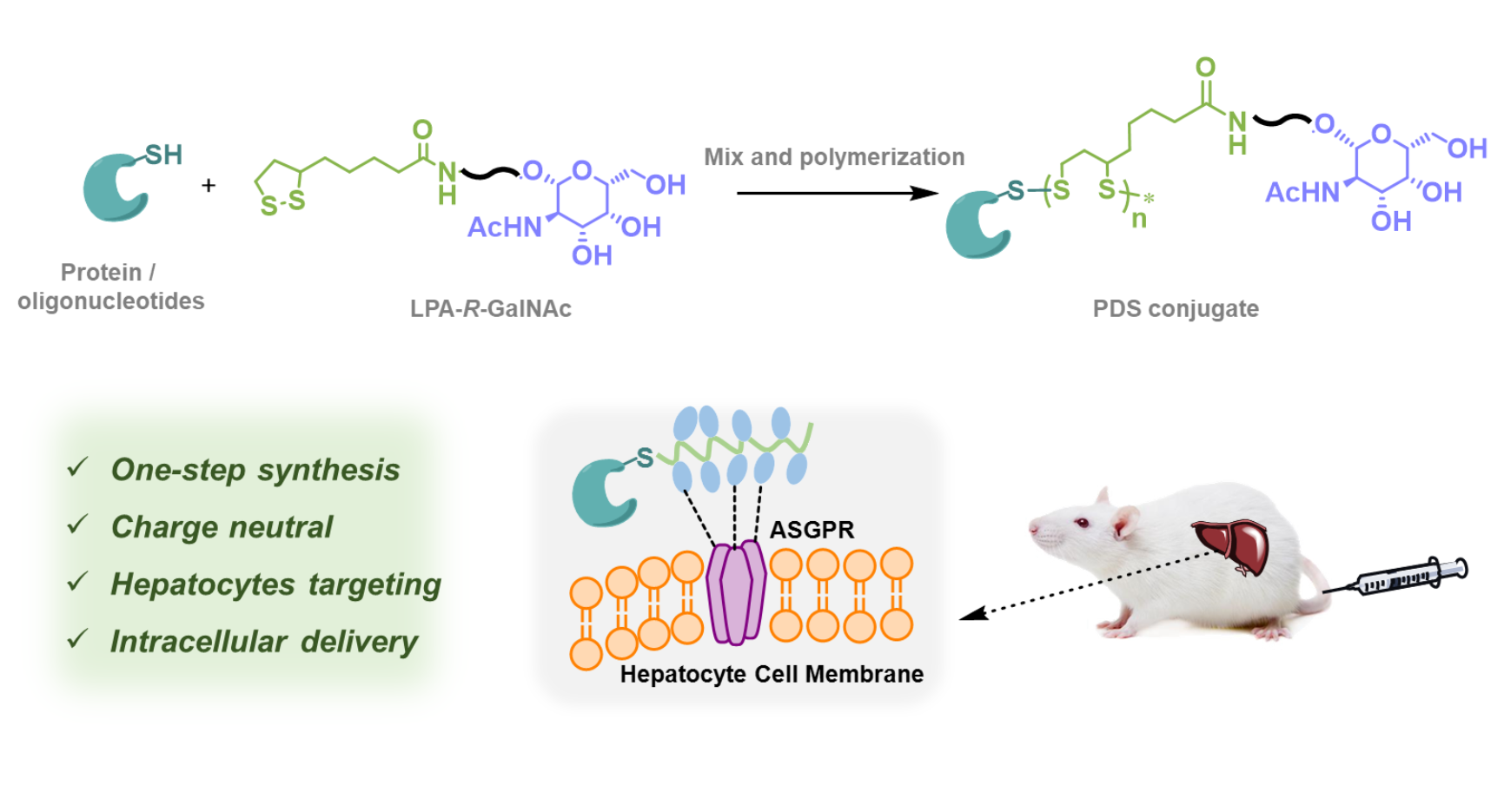
Biologics, including proteins and antisense oligonucleotides (ASOs), face significant challenges when it comes to achieving intracellular delivery within specific organs or cells through systemic administrations. Recently, the group of Prof. Hua Lu at the College of Chemistry and Molecular Engineering, Peking University developed a novel approach for delivering proteins and ASOs to liver cells, both in vitro and in vivo, using conjugates that tethering N-acetylated galactosamine (GalNAc)-functionalized, cell-penetrating polydisulfides (PDS). The method involves the thiol-bearing cargo-mediated ring-opening polymerization of GalNAc-functionalized lipoamide monomers through the so-called aggregation-induced polymerization, leading to the formation of site-specific protein/ASO-PDS conjugates with narrow dispersity. The hepatocyte-selective intracellular delivery of the conjugates arises from a combination of factors, including firstly GalNAc's binding with ASGPR receptors on liver cells, leading to cell immobilization, and the subsequent thiol-disulfide exchange occurring on the cell surface, promoting internalization. Their findings emphasize the critical role of the close proximity of the PDS backbone to the cell surface, as it governs the success of thiol-disulfide exchange and, consequently, cell penetration. These conjugates hold tremendous potential in overcoming the various biological barriers encountered during systemic and cell-specific delivery of biomacromolecular cargos, opening up new avenues for the diagnosis and treatment of a range of liver-targeting diseases.
This work was supported by the National Key Research and Development Program of China, National Natural Science Foundation of China, Beijing Natural Science Foundation, and Beijing National Laboratory for Molecular Sciences.
Original link for the paper: https://pubs.acs.org/doi/10.1021/jacs.3c11914