Water Activation and Hydrogen Production

Water is the biggest pool in terms of hydrogen supply for the future. Polymer electrolyte membrane fuel cells (PEMFC) and other applications that run on hydrogen are the most promising alternatives to meet the future needs for power supply in portable, residential and transportation applications. These applications rely on safe storage and transportation of hydrogen. Hydrogen stored in a chemical form as liquid organic compounds and released in-situ on demand through efficient catalytic processes at low temperature is highly desirable. For example, hydrogen could be stored in ammonia through ammonia synthesis process in hydrogen-abundant area, and transported and released through ammonia decomposition. Also, methanol, an inexpensive bulk chemical, can reform with water and produce three hydrogen molecules (CH3OH + H2O = 3H2 + CO2) with high gravimetric density of 18.8 wt%. We are working on those processes in our laboratory.
Catalytic Upgrading/Degradation of Plastic Wastes
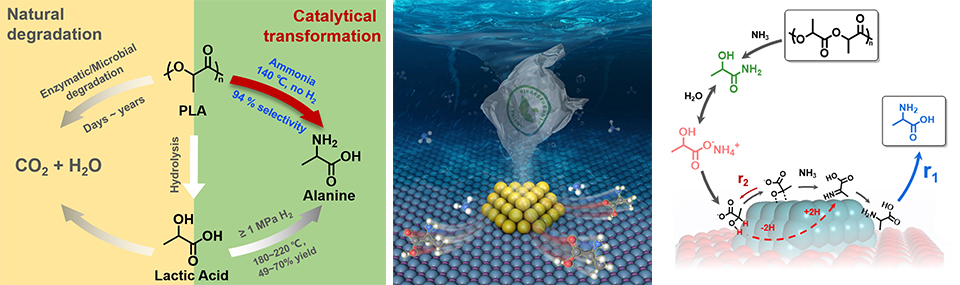
The increasing use of polymer plastics and the accumulation have posed a huge threat to the environment. Transforming the plastic wastes into value-added chemicals is a promising way to recycle the carbon resources and to reach carbon neutrality. However, most of the demonstrated catalytic degradation/upgrading processes suffer from insufficient selectivity toward a specific product due to the stubborn structure and random fission of the polymer chains, especially for polyolefins. We aim at establishing novel catalytic processes (e.g., new reaction routes, new catalysts for high selectivity of specific products, new degradation reagent, etc.) to produce value-added chemicals from real-life plastics. While paving the road for these processes, the poisoning effect from the additives (e.g., plasticizer, stabilizer, antioxidant and chlorine-containing components) and the complexity of different polymer components in the plastic wastes are the major challenges we strive to break through.
C1 Chemistry

C1 chemistry is the most promising alternative to petroleum route for the production of fuels and basic chemical raw materials. Through C-O and C-H bond activation and successive C-C, C-O and C-H bond formation, one carbon-atom molecules, such as CO, CO2 and CH4, can reassemble to large molecular weight hydrocarbons, oxygenates, and other important chemicals on designated catalysts. We strive to find new catalytic systems for existing C1 transformation processes, develop new reaction paths for more efficient utilization of those C1 resources, and reveal their reaction mechanism by various characterization methods under working reaction conditions.
Operando Reaction Mechanism and Kinetics

Although heterogeneous catalysis as a phenomenon has been studied for over two hundred years, attempts to describe the reactions at the molecular level have faced significant challenges. A predominant barrier for researchers to enhance their knowledge in this area is the lack of powerful tools that can track the catalytic reactions under working conditions, especially for those occurring at elevated pressure and/or temperature. It is worth noting that some sophisticated in-situ methods have been developed in order to identify the working catalyst phases (active centers) responsible for catalytic activity or detect the reaction intermediates and eventually to reveal how the reactants are converted into the products (reaction mechanism). State-of-the-art technologies even enable the resolution of the spatiotemporal gradients in heterogeneous catalysts, and in some cases, imaging the heterogeneity of catalytic active components even at the nanometer scale. However, for high-pressure reactions, such as ammonia synthesis and Fischer-Tropsch synthesis (FTS), limited approaches are available for direct observation of the reaction process under working conditions. We designed and manufactured a series of operando apparatuses for solid-state-NMR, X-ray adsorption, X-ray diffraction as well as Steady-State Isotopic Transient Kinetic Analysis (SSITKA) that could work at elevated pressure and temperature. Coupling them with on-line Mass Spectroscopy, GC-Mass, and even high pressure transient technology, we are able to provide insights into mechanistic understanding of chemical reactivity of different complex reaction systems. Those methods have also been used to monitor the catalyst fabrication process, which is key for the controlled construction of catalytic-active materials.
Catalyst Fabrication
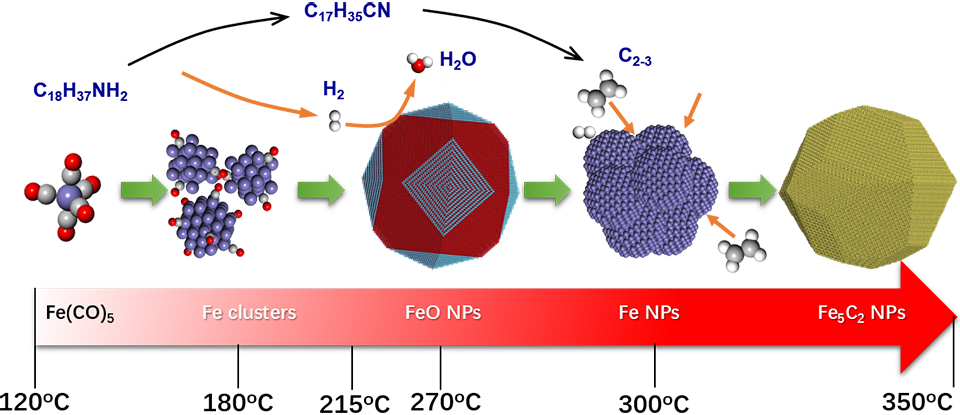
The production of most industrially important chemicals involves catalysis. The chemical compositions, particle sizes, morphology, porosity, exposed facets as well as defects and interfaces of the catalysts have significant effects on their catalytic performances. Therefore, catalyst design is the center-of-art of the scene. The right choice of the above mentioned measures for catalyst will confer it designated electronic and geometrical structures and therefore designated catalytic properties. Our mission is to discover the relationship between the catalyst structure and its catalytic performance. Under this consideration, we focus our research activities on the controllable synthesis of well-defined catalytic materials, and the investigation of their catalytic performances. Meanwhile, we work closely with our collaborators on materials research to construct the most active and selective catalysts, especially for low temperature applications.