Research news, Mar. 28th, 2018
Liang Liu, Miaomiao Zhu, Hai-Tao Yu, Wen-Xiong Zhang, and Zhenfeng Xi*
Organometallics 2018, 37, 845-847.
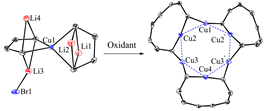
Organocopper(I) compounds, mainly organocopper(I) aggregates and organocuprates(I), have long been attractive both synthetically and structurally. Remarkable progress has been made to reveal the structures of organocopper(I) aggregates, in which trigonal, square and pentagonal core Cun (n = 3-5) structures have been reported. However, their hexanuclear analogues remain less explored, although several copper(I) hydride and carboxylate complexes are known. On the other hand, as a synthetic strategy, oxidative coupling of organocuprates(I) are efficiently utilized to construct C-C or C-heteroatom bonds, via a process in which copper(III) complexes and organocopper(I) aggregates are often proposed as key intermediates (for our recent related work, see: Liang Liu, Miaomiao Zhu, Hai-Tao Yu, Wen-Xiong Zhang, and Zhenfeng Xi, Organocopper(III) Spiro Complexes: Synthesis, Structural Characterization, and Redox Transformation, J. Am. Chem. Soc. 2017, 139, 13688-13691).
In this work, spiro butadienyl tetraorganocuprates were synthesized from butadienyl dilithio reagent and 0.5 equiv of copper(I) salt. Oxidation reaction of these spiro butadienyl tetraorganocuprates with I2 or p-benzoquinone afforded an octatetraenyl organocopper(I) aggregate. This result demonstrated that the butadienyl skeletons rather than the copper(I) atom were oxidized preferentially. Single crystal X-ray structural analysis revealed that the high-order organocuprates have linear or zigzag polymeric structures, while the hexanuclear octatetraenyl organocopper(I) aggregate consists of a core Cu6 structure, which forms a regular hexagon. These results demonstrate that either the organic skeletons or the copper(I) atom of organocuprates(I) can undergo oxidation reaction, which is fundamentally helpful for understanding the mechanism of oxidation reaction of organocuprates.
