Dinitrogen activation by a titanium hydride complex supported by 2-butene ligand
Xianghui Shi, Yongliang Zhang, Mingdong Zhong, Rui Feng, Yuanjin Chen, Lei Yu, Yue Wu, Junnian Wei,* Zhenfeng Xi*
Inorg. Chem. Front., 2024. DOI: 10.1039/D3QI01911J.
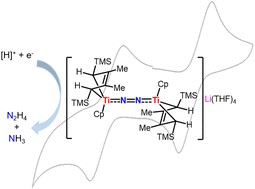
The activation of dinitrogen by a titanium hydride complex with two bridging hydrides located between metal Ti and Li was investigated. Exposing cyclic bis-alkylidene titanium complex 1 to H2 in THF solution yielded titanium hydride 2, {[(2-butene)(C5H5)Ti](μ-H)2Li(THF)3}. 2 aggregated into dimer 3 in hexane, accompanied by the release of LiH. Treatment of 2 with N2 (1 atm) in THF resulted in ionic end-on bridged dinitrogen dititanium complex 4, {[((2-butene)(C5H5)Ti)2(μ2-η1,η1-N2)][Li(THF)4]}, without using external reducing agents. Furthermore, 4 could be oxidized to yield the binuclear complex 5, {[(2-butene)(C5H5)Ti]2(μ2-η1,η1-N2)}. The solid-state structures of 4 and 5, as revealed by X-ray crystallographic studies, exhibit relatively short N–N bond distances of 1.198(2) Å and 1.179(2) Å, respectively. These results align with the observed N–N Raman stretching frequencies at 1699 cm−1 and 1704 cm−1. The N2 moiety bridging two titanium atoms could be converted into ammonia and hydrazine upon treatment with a proton source and reductant.