Metallic Chemical Biology (Bioinorganic Chemistry) is a frontier at the intersection of inorganic chemistry and life sciences, we focus on developing and using neotype inorganic compounds as exogenous metal molecular tools to detect or regulate the process and dynamics of important molecular events in life sciences in real-time, in situ and quantitatively. We are committed to bridging the gap between inorganic chemistry and life sciences by constructing novel metal (rare-earth) molecular tools through natural metalloenzyme simulations, and expanding the applications in the visualization of molecular events in life processes and the regulation/modification of important molecules from both metal luminescence and reaction aspects, providing new markers and new drug lead structures for the diagnosis and treatment of major diseases.
1. Natural Metalloenzyme Simulations and Porpholactone Chemistry
Tetrapyrrole metal coenzyme is the active center of photosynthetic system, oxidase, etc., which converts visible/near-infrared light into electrical/chemical energy. Porpholactone was used as a molecular platform to construct a bionic tetrapyrrole ligand library, and systematically simulate the regulation of luminescence and catalysis by structural factors such as conjugation degree and molecular symmetry, so as to solve two important chemical problems. One is regioisomerism. Natural tetrapyrrole metal coenzymes (such as chlorophylls) fine-tune their light absorption behavior to adapt to their environment (such as deep sea and land) through regional isomerism, i.e. the relative changes in the positions of two lactone groups within the conjugate macroring.
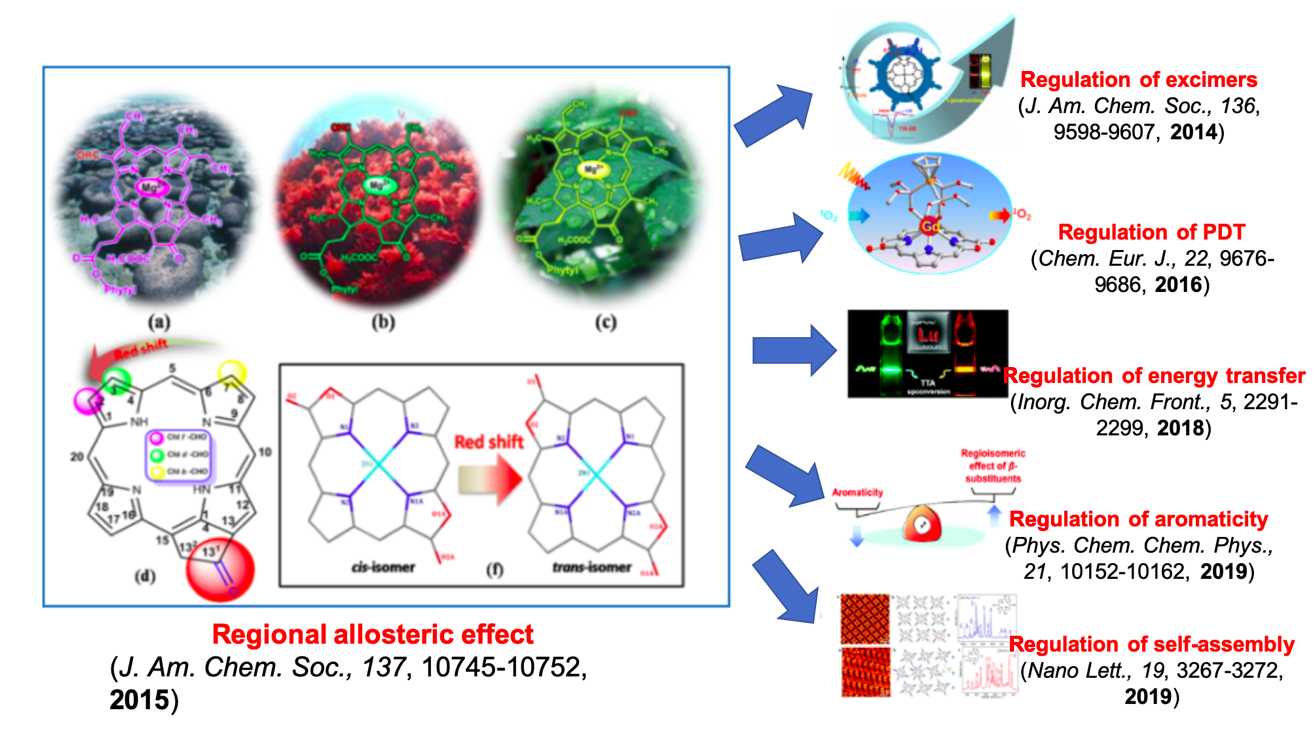
The second is to reveal the electronic structure and metal reactivity related to saturation. Take nickel chlorophyll (tunichlorin) for example. In 1989, Professor Rinehart in the United States found nickel chlorophyll in the capsule organisms, but the structure is unstable. It has long been believed that the formation of nickelchlorophyll is the production of magnesium ions that replace chlorophyll after the decomposition of the nickel cluster of hydrogenase in photosynthesis, so nickelchlorophyll is the "nickel ion recycle station". Porpholactone was used as a model molecule to investigate the significant improvement of oxidation-reduction reactivity (HYDROGEN evolution) of nickel metal by multiple synergistic effects such as aromaticity and secondary coordination environment. The reaction rate was about 200 times faster than that of porphyrin analogue. By simulating the structure-activity relationship, we first proposed and verified the importance of chlorophyll structure to the catalysis and electron transfer function of nickel, breaking through the traditional understanding of "nickel ion recycling station", understanding the photosynthetic system and biomimetic molecular catalysis.

Chem. Sci. 2017, 8, 5953-5961; ACS Catalysis 2020, 10 , 2177-2188; highlighted by Nat. Rev. Chem. 2017 1 , 0062,
2. the Chemical Biology of Rare Earth
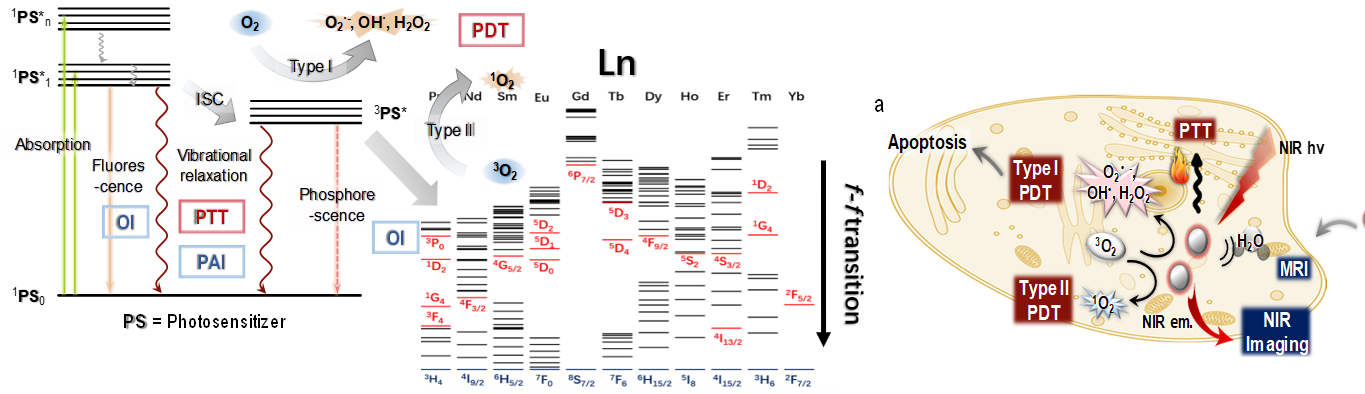
The luminescence of rare earth elements is mainly based on the electron transition of f orbital, and all rare earth elements are covered by luminescence from UV-visible to near-infrared region.By replacing the rare earth species and customizing probes with specific luminescence wavelength, photochemical reaction characteristics, etc., luminescent rare earth complexes (rare earth molecular toolbox) with "element characteristics" from visible to near-infrared regions are established. Based on the optimization of imaging function, "match" ligand excitation state and rare earth energy level (f-f transition), realize the activation of singlet oxygen and near-infrared (or MRI) imaging, and use "image-mediated" method to treat diseases with spatio-temporal accuracy. To develop the biocompatibility modification of rare earth probe, improve the biological safety of rare earth, expand the therapeutic function and deepen the chemical biology of rare earth.
3. Metallic Pharmacochemistry and its Clinical Transformation
Metal chemical and biological studies show that metal complex probes play an important role in the treatment and diagnosis of diseases. Due to its unique electron configuration, coordination environment and geometric structure, metal probes have multiple targets and complex mechanisms, similar to the geometric size of organic small molecules, but their modes of action are significantly different. The biological activity of metal complexes is highly dependent on the thermodynamics (the strength of metal-ligand bonds), dynamics (the time scale of the formation and fracture of ligand bonds), metal oxidation state and molecular morphology (intermolecular interaction) of the coordination process. Therefore, the design of the metal molecular probe will track the metal uptake, location, morphological changes and other processes at the cellular/in vivo level, so as to realize the visualization of the target and obtain the treatment feedback information in the shortest time, which is of great significance for the efficacy monitoring, evaluation and precision treatment.

J. Am. Chem. Soc. 2020, 142, 22