Recent Highlights
Contact us
张文彬课题组
地址:北京市海淀区成府路202号
太阳成集团tyc9728
邮编:100871
电话:010-62766876
电邮:wenbin@pku.edu.cn

请扫以上二维码关注我们课题组的公众号。
我们将定期推送组会每周精读和泛读文献介绍以及课题组的最近新闻!
--------------------------------------------
News
Congratulations to Fang Jing's feature article published in Acta Polymerica Sinica!
DOI: 10.11777/j.issn1000-3304.2018.18034
See: http://www.gfzxb.org//article/doi/10.11777/j.issn1000-3304.2018.18034
Jing Fang, Wen-bin Zhang. Genetically Encoded Peptide-protein Reactive Pairs[J]. Acta Polymerica Sinica, 2018, (4): 429-444.
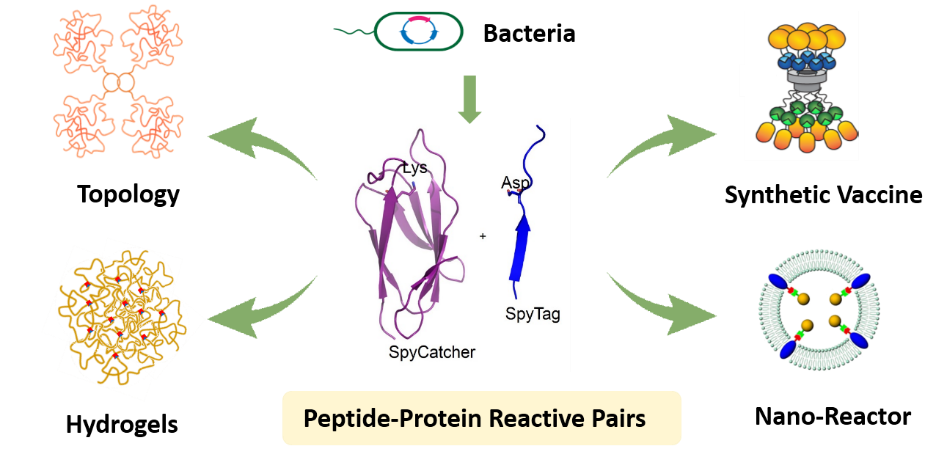
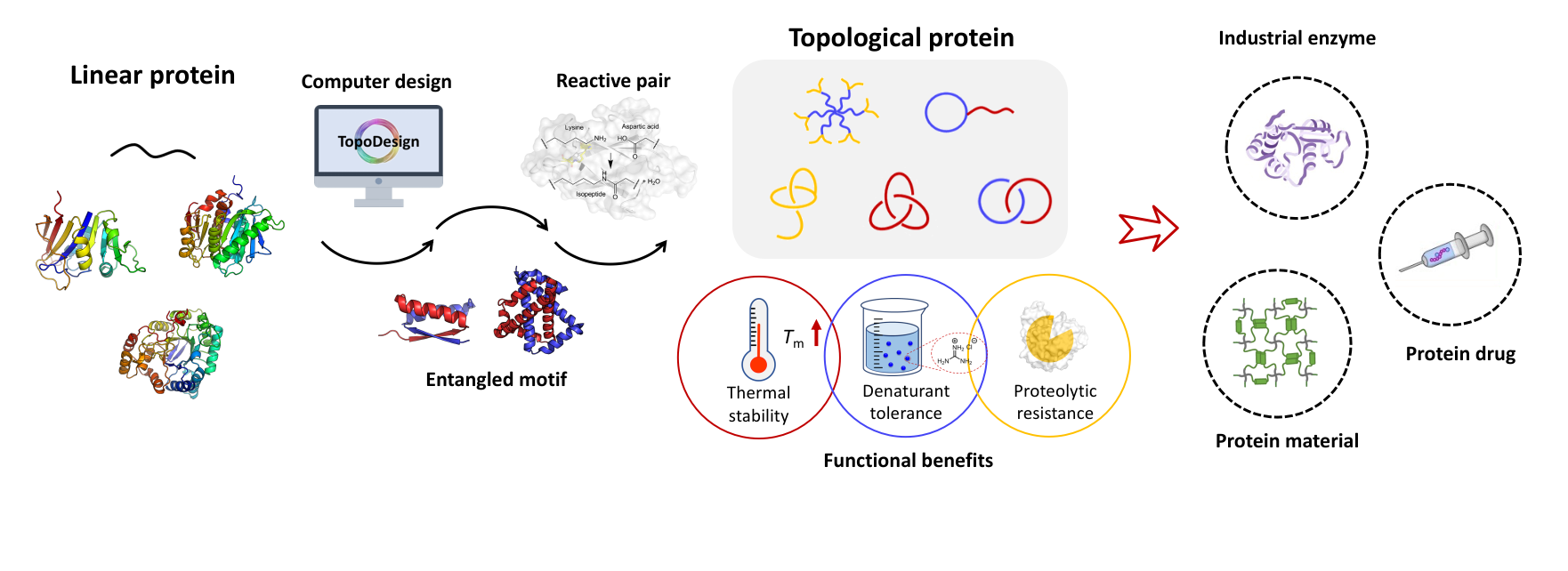
Chemical modification of proteins is of great significance in protein engineering, biomaterials, and chemical biology. Genetically encoded peptide-protein reactive pair, or “molecular superglue”, refers to a peptide tag and its protein partner that can spontaneously reconstitute to form an isopeptide or ester bond between the side chains of specific residues of the two components. It is entirely based on natural amino acids and thus genetically encodable, providing a new way to do chemistry with proteins. This feature article summarizes the development of this unique set of chemical tools and elaborates on the principles and mechanisms of the isopeptide formation as well as the application of these tools in diverse fields. To date, a toolbox of peptide-protein reactive pairs has been developed and gradually gained its popularity in various fields such as protein topology engineering, protein-based biomaterials and protein nano-assemblies. Typical pairs include the isopeptide-bond-forming SpyTag/SpyCatcher, SnoopTag/SnoopCatcher, SdyTag/SdyCatcher etc. and the ester-bond-forming Cpe0147-A/ Cpe0147-B. It allows the programming of post-translational modification of nascent proteins in vivo, which, in combination with protein folding, leads to versatile nonlinear protein topologies with unique properties, including circular proteins, star proteins, and protein catenanes. The protein catenation is found to enhance both the stability and the activity of the enzyme like dihydrofolate reductase. Their reactivity in vitro is also excellent. The covalent nature of SpyTag/SpyCatcher interaction has facilitated the processing of proteins into various materials forms including all-protein-based, chemically cross-linked hydrogels, functional layer-by-layer thin films, hybrid colloidal assemblies, and “living” materials. In this sense, they can serve as the “iron grip” to bring two parts together to form the conjugate, which may be helpful for diverse purposes such as the sortase activity enhancer. It also allows the preparation of protein nano-assemblies with ultra-high affinity, which are useful for applications like protein nanoreactors, synthetic vaccines, and protein therapeutics. The peptide-protein reactive pair technique thus opens new horizon in protein chemistry and paves the road to the synthesis and application of precision macromolecules with huge potential in real applications.
蛋白质的化学修饰在蛋白质工程、生物材料以及化学生物学等领域具有重要的意义. 近年来,可基因编码的多肽-蛋白质化学反应对的发展为蛋白质的化学修饰提供了新的思路和强大的工具. 本专论回顾了此类新型化学方法的发展背景,详细阐述了多肽-蛋白质反应对的作用原理和调控机制,介绍了业内在拓展一大类具有各种特性的反应对家族方面的初步尝试,并总结了它们在蛋白质拓扑工程学、蛋白质材料以及蛋白纳米组装体等诸多方面的应用. 蛋白质独特的可基因编码的特性赋予了其广泛的可修饰性和体内应用的潜力,而由其衍生的诸多蛋白质超分子结构更为发展人工蛋白质机器以及多功能的“活”材料奠定了基础.
本专论得到了国家自然科学基金委、科技部863计划和青年千人计划的支持,在此表示衷心的感谢!特别感谢《高分子学报》的邀请,给了我们展示这方面工作的机会!