Quotes
在科学上没有平坦的大道,只有那些不畏艰险沿着陡峭山路攀登的人,才有希望达到光辉的顶点。
----马克思
-----------------------------------------------
Research Projects
Collaborations
请有兴趣的研究组联系我们。欢迎任何形式的合作,尤其是在自组装、水凝胶以及生物医药等方向的合作。
------------------------------------------
Publications
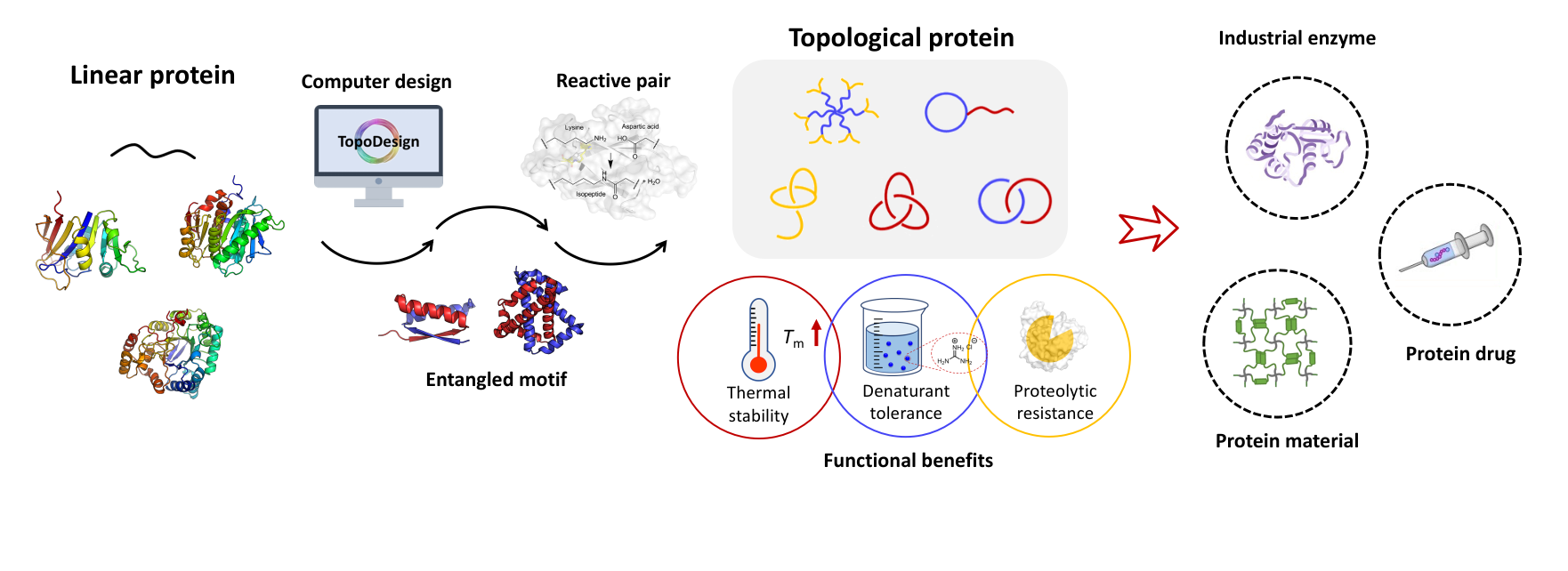
Zhang, X.-J.;# Wu, X.-L.;# Wang, X.-W.; Liu, D.; Yang, S.;* Zhang, W.-B.* SpyCatcher-NTEV: A Circularly Permuted, Disordered SpyCatcher Variant for Less Trace Ligation. Bioconjug. Chem. 2018, 29, 1622-1629
ABSTRACT: The SpyTag/SpyCatcher reaction has emerged as a powerful way for bioconjugation, but it leaves a folded complex in the product after the formation of the isopeptide bond. To vary the location of the reactive residue and reduce the size of the complex and its potential immunogenicity, we engineer two circularly permuted SpyCatcher variants, SpyCatcher-N and SpyCatcher-NTEV, the latter of which possesses a TEV-recognition site for removal of the fragment containing the catalytic site. Surprisingly, both variants are found to be disordered in solution, yet still retain the ability to form an ordered complex upon reaction with SpyTag with secondorder rate constants of ∼10 M−1 s−1. Cellular expression of a telechelic protein bearing SpyCatcher-NTEV at the N-terminus and SpyTag at the C-terminus gives both cyclized and chain-extended products. Notably, the monomers exist almost exclusively in the cyclic form owing to its high reactivity in vivo. The fragment containing the catalytic site of SpyCatcher-NTEV can then be removed by TEV digestion, giving a circular protein with minimal trace from the ligation reaction. The plasticity of SpyTag/ SpyCatcher reactive pair has promised an ever-expanding toolbox of genetically encoded peptide−protein reaction with versatile features.