最新热点
联系我们
张文彬课题组
地址:北京市海淀区成府路202号
太阳成集团tyc9728
邮编:100871
电话:010-62766876
电邮:wenbin@pku.edu.cn

请扫以上二维码关注我们课题组的公众号。
我们将定期推送组会每周精读和泛读文献介绍以及课题组的最近新闻!
--------------------------------------------
最新成果
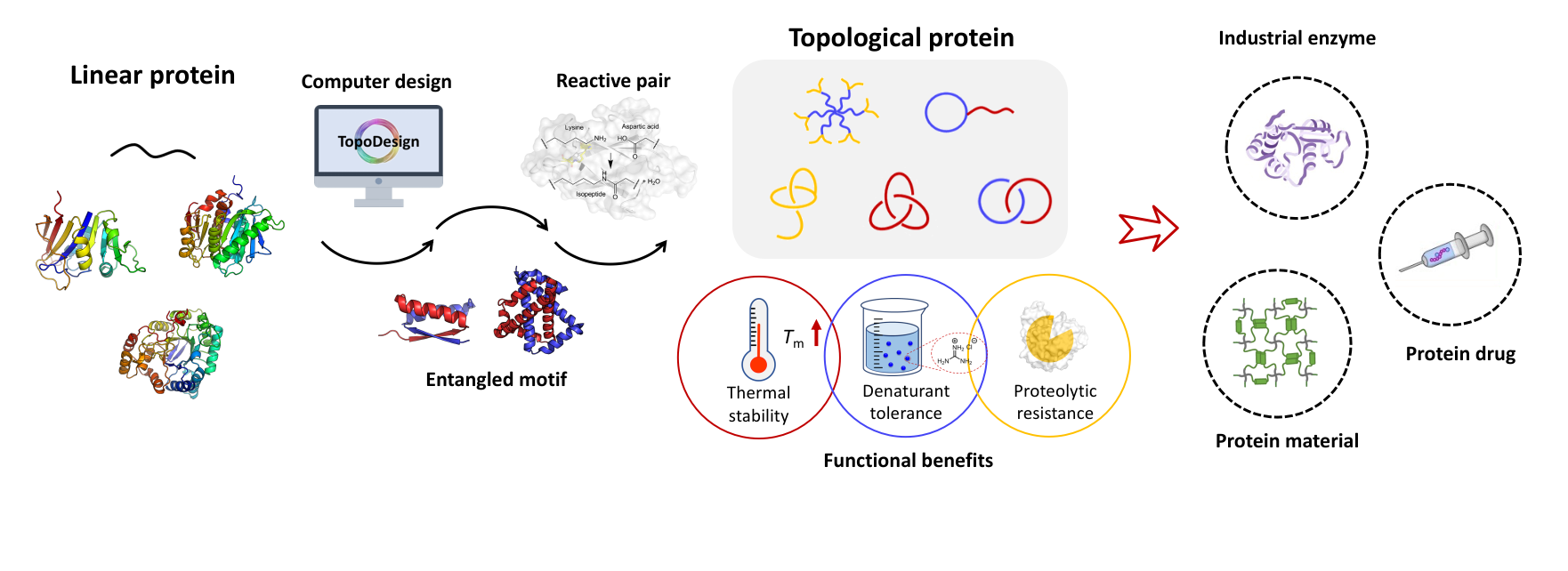
Fang, J.; Li, T.; Lee, J.; Im, D.; Xu, L.; Liu, Y.; Seo, J.; Zhang, W.-B.* A single-domain protein catenane of dihydrofolate reductase. Natl. Sci. Rev. 2023, 10, nwad304. https://doi.org/10.1093/nsr/nwad304

A single-domain protein catenane refers to two mechanically interlocked polypeptide rings that fold synergistically into a compact and integrated structure, which is extremely rare in nature. Herein, we report a single-domain protein catenane of dihydrofolate reductase (cat-DHFR). The design was achieved by rewiring the connectivity between secondary motifs to introduce artificial entanglement and the synthesis was readily accomplished by a series of programmed streamlined post-translational processing events in cells without any additional in vitro reactions. The target molecule contains few exogenous motifs and has been thoroughly characterized by combined techniques of LC-MS, SDS-PAGE, protease cleavage experiment, and ion mobility mass spectrometry. Compared to the linear control, cat-DHFR retains the catalytic capability and exhibits enhanced stability against thermal or chemical denaturation due to conformational restriction. The results suggest that linear proteins may be converted into concatenated single-domain counterparts with almost identical chemical composition, well-preserved function, and elevated stability, which represents an entirely new horizon in protein science.